
Researchers at the University of Southern California have found a way to make an effective and competitive redox flow battery out of the iron industry’s waste products. Luckily for us, the results of the paper were posted on an open journal and we could take a look into the tech behind this battery.
As electric utilization, adoption of electric cars, and the use of renewable power continues to rise, engineers all over are searching for the perfect utility scale battery. We have all heard about Tesla’s 100MW lithium battery pack in South Australia. The system is a massive success and has already paid itself back. However, engineers all over were quick to point out that, until we have a breakthrough, Lithium cells are just not the right choice for a utility system in the long run. There has to be a better solution.
WHAT SHOULD A GOOD BATTERY LOOK LIKE?
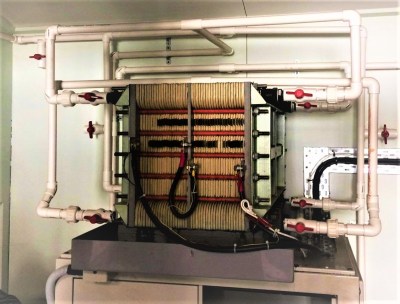
Grid scale storage is a important and difficult problem to solve. If we don’t have batteries, we can’t move away from fossil fuel power generation. Without grid storage every power surge, such as England turning on its kettles for tea, would knock it out.
Currently power plants are spooled up and down all day to match the demand. Even if we didn’t have renewable energy on the horizon, better batteries would allow us to run smaller power plants more efficiently to service demand. You could run one plant at maximum efficiency all day and the battery could level the load. It’s the difference between your car’s fuel economy if you slowdown and accelerate in the city or stay at cruising speed on the highway.
The ideal battery has a few requirements. It has to take charge at a reasonable rate. It also needs to be able to delivery that charge instantly. Most importantly, the battery must be cheap and last a long time. It’s literally the impossible fast, good, cheap dilemma. Engineers have tried everything from molten salt to stacking rocks. A hydroelectric dam, for example, is nothing more than a battery made out of water and gravity. Tesla’s large batteries are made out of lithium-ion cells.
The primary economic decision driver in picking a system like this is the Levelized Cost of Energy Storage (LCOS). This is the sum of the capital and operating costs of the system by the total energy stored and delivered over the life of a system. The paper mentions that the US Department of Energy specifies an LCOS target of 2.5 cents / KWh. At an installation cost of $200 / kWh, a battery must deliver 8000 kWh of energy over its lifetime. As the authors point out, if you consider one discharge and charge cycle a day, this means the battery should last 22 years. We know that lithium cells are not up to that challenge.
REDOX FLOW BATTERIES
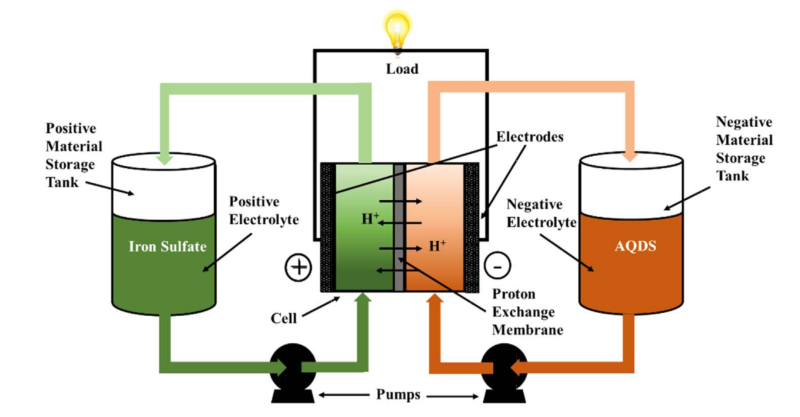
There is another type of battery out there, which we’ve covered before, called a redox flow battery. This battery has the potential for nearly unlimited life, low operating cost, and might even be ecologically friendly.
In this battery two chemicals flow past each other separated by a membrane. Depending on how you place the electrodes in the chemicals, protons pass through the membrane from one vat to another building up a potential difference between the two. The substances used can be the same chemicals, different ones, or even mixes of the two.
The current state of the art redox battery is based on vanadium. The largest install is in Japan at 60 MWh. However, China’s truly impressive power-infrastructure super-corporations are currently building a 800 MWh install. However, vanadium isn’t cheap, and the material is more than a little bit toxic.
IRON BATTERIES
There’s another element that works wonderfully in these batteries, iron. It’s just waiting on a breakthrough that would let it operate at scale, and that’s where the researchers have had their breakthrough. They found that they can use iron sulfate, a by-product of the iron industry, and anthraquinone disulfonic acid as the chemical on both sides of the vat and get a very effective redox battery.
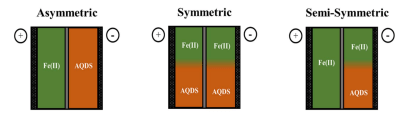
It has every advantage over the vanadium battery aside from a lower cell voltage. This means that the battery installation has to be a bit larger and more complex, but in the end it stands to be cheaper because the primary electrolytic is a relatively safe industrial offshoot, and the battery has the simplicity of it being a symmetric system: the chemical mix on both sides of the membrane is the same, making refreshing the solution easy. The authors estimate that this battery will cost $54 / kWh while the current state of the art vanadium batteries are hovering between $160 / kWh to 180 / kWh. That’s a nice reduction.
These batteries are fascinating bits of technology. They’re also surprisingly hackable. Of course some of the steps to get maximum efficiency, such as doping the membrane with carbon nano-tubes may require [Ben Krasnow] levels of hacking. But rudimentary batteries can be made out of scrap, which is a pretty good indication of the technology’s viability. We’re curious to see how energy storage revolutions will change the world in the future. What do you think?
www.hackaday.com

