Date: Oct 8, 2018
Abstract
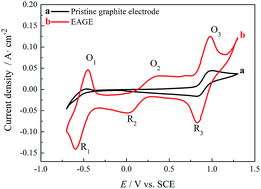
An electrochemically activated graphite electrode (EAGE) was obtained by a simple and moderate method of anodic potentiostatic polarization. The composition, microstructure, and electrochemical properties of the EAGE were characterized by high-resolution X-ray photoelectron spectroscopy, high-resolution transmission electron microscopy, cyclic voltammetry and electrochemical impedance spectroscopy. The results show that the electrochemical activity and the reversibility for electrode processes of V(II)/V(III) and V(IV)/V(V) couples on the EAGE are significantly improved due to the introduced C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and COOH groups. The rate constant of charge transfer for the anodic oxidation of V(IV) on the EAGE was determined to be 8.17 × 10−4 cm s−1, which is about 20 times larger than the 4.09 × 10−5 cm s−1 rate constant on the pristine graphite electrode.
O and COOH groups. The rate constant of charge transfer for the anodic oxidation of V(IV) on the EAGE was determined to be 8.17 × 10−4 cm s−1, which is about 20 times larger than the 4.09 × 10−5 cm s−1 rate constant on the pristine graphite electrode.